Wikipedia:POTD column/June 28, 2006
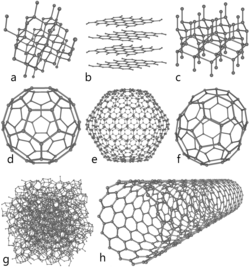
Carbon is a chemical element in the periodic table that has the symbol C and atomic number 6. It occurs in all organic life and is the basis of organic chemistry. This nonmetal also has the interesting chemical property of being able to bond with itself and a wide variety of other elements, forming nearly 10 million known compounds. This illustration depicts eight of the allotropes (different molecular configurations) that pure carbon can take (top to bottom, left to right): a) Diamond, b) Graphite, c) Lonsdaleite, d) Buckminsterfullerene (C60), e) C540, f) C70, g) Amorphous carbon, h) single-walled carbon nanotube.
Image credit: Michael Ströck
Archive - More featured pictures...